上海金畔生物科技有限公司为生命科学和医药研发人员提供生物活性分子抑制剂、激动剂、特异性抑制剂、化合物库、重组蛋白、同位素标记物,专注于信号通路和疾病研究领域。
5-Fluorouracil-d1 (Synonyms: 5-FU-d1)
5-Fluorouracil-d1 (5-FU-d1) 是 5-Fluorouracil 的氘代物。5-Fluorouracil (5-FU) 是一种尿嘧啶的类似物 (nucleoside antimetabolite/analog),是一种有效的抗肿瘤药。5-Fluorouracil 通过抑制胸苷酸合成酶影响嘧啶的合成,从而耗尽细胞内dTTP 池。5-Fluorouracil 诱导细胞凋亡 (apoptosis),可用作化学敏化剂。5-Fluorouracil 还可抑制 HIV 病毒。5-Fluorouracil (5-FU) 可破坏外泌体特异性的 rRNA。
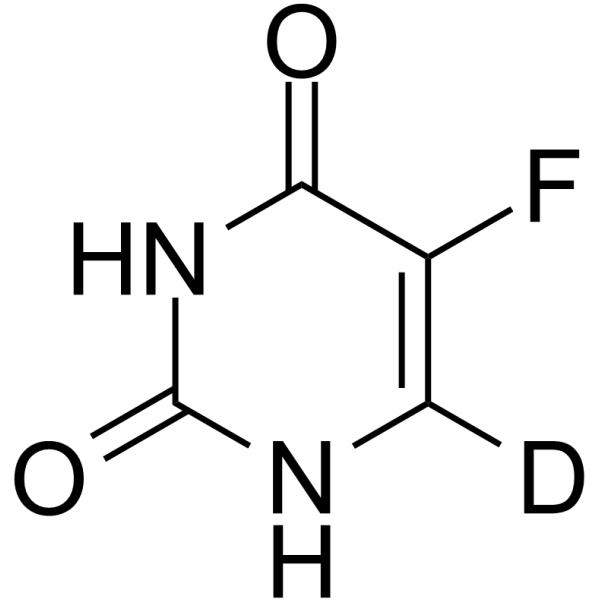
5-Fluorouracil-d1 Chemical Structure
CAS No. : 90344-84-6
| 规格 | 是否有货 | ||
|---|---|---|---|
| 5 mg | Check price and availability | ||
| 50 mg | Check price and availability | ||
* Please select Quantity before adding items.
| 生物活性 |
5-Fluorouracil-d1 (5-FU-d1) is the deuterium labeled 5-Fluorouracil. 5-Fluorouracil (5-FU) is an analogue of uracil and a potent antitumor agent. 5-Fluorouracil affects pyrimidine synthesis by inhibiting thymidylate synthetase thus depleting intracellular dTTP pools. 5-Fluorouracil induces apoptosis and can be used as a chemical sensitizer[1][2]. 5-Fluorouracil also inhibits HIV[3]. |
|---|---|
| 体外研究 (In Vitro) |
Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
| 分子量 |
131.08 |
| Formula |
C4H2DFN2O2 |
| CAS 号 |
90344-84-6 |
| 中文名称 |
5-氟脲嘧啶 d1 |
| 运输条件 |
Room temperature in continental US; may vary elsewhere. |
| 储存方式 |
Please store the product under the recommended conditions in the Certificate of Analysis. |
| 参考文献 |
|
所有产品仅用作科学研究或药证申报,我们不为任何个人用途提供产品和服务
